3D-CCM – Three-dimensional in vivo microscopy of the cornea for the diagnosis of Ocular Surface Diseases
- Contact:
Dr. Bernd Köhler
- Project Group:
System Integration - Inspection and Digital Image Processing (SI-IIP)
- Funding:
DFG
- Partner:
Rostock University Medical Center
- Startdate:
2019
- Enddate:
2022
The integrity of the corneal surface – particularly the epithelium – is maintained by the functional relations between cornea, conjunctiva, limbus, and their interconnections with the tear film and lid apparatus. Disorders arising from disturbances of this balance are collectively termed Ocular Surface Diseases (OSD). OSD may be caused by tear secretion malfunctions, scar formation following infectious or non-infectious inflammation or thermal/chemical trauma, but also by diabetes mellitus, limbal stem cell deficiencies or Meibomian gland dysfunction.
Diagnosis of OSD plays an essential role in the development of cause- and patient-specific treatment strategies. Currently established methods allow for the macroscopic description of corneal alterations, but not for quantitative characterization on a cellular level due to insufficient resolution. The only available method that provides non-invasive access to these cellular areas is in vivo corneal confocal microscopy (CCM). However, quantification of cellular alterations is currently restricted to 2D analyses with small fields of view, hardly location-specific, and often requires extensive manual postprocessing. Furthermore, the surface-parallel image formation does not correspond to the axial images of slit lamp examinations that ophthalmologists use on a daily basis. With these constraints, CCM is rarely implemented in clinical routines.
The proposed project addresses these methodical deficits and develops high resolution, wide-field 3D imaging technologies and methods for the volumetric morphometric characterization of epithelial cell structures. Automated 3D imaging techniques ensure the reliable acquisition of an extended volume while simultaneously minimizing acquisition duration. Online visualization in arbitrary image planes will enable the ophthalmologist to qualitatively assess the corneal tissue or the dimension of corneal defects in real-time. Following the imaging process, a high-precision volume representation of the entire recorded tissue region will be reconstructed from the 2D image datasets, providing the basis for a reliable morphometric analysis of the corneal tissue. From the reconstructed volumes, volumetric features with 3D location information will be calculated for the epithelial tissue and the adjacent subbasal nerve plexus.
The proposed project builds on the DFG project “Automated method for large-area imaging of the corneal sub-basal nerve plexus as basis for a reliable biomarker for the assessment of diabetic neuropathy” and offers a novel approach for a volumetric characterization of the corneal tissue on a cellular level that can advance clinical diagnostics and further the development of cause- and patient-specific treatment strategies for OSD. The novel 3D imaging techniques and visualization of arbitrarily oriented image planes will lay the foundations for high-resolution quantitative slit lamp microscopy.
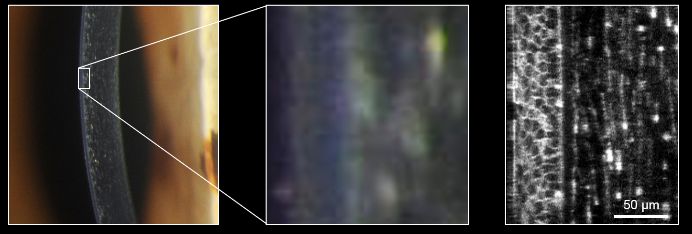
Left and center: slit lamp microscope. Right: confocal microscope.
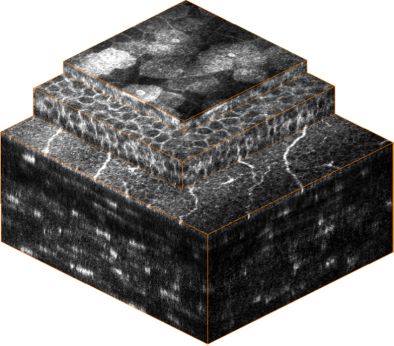
From top to bottom: Epithelium, sub-basal nerve plexus, stroma.
Team:
Dr. Bernd Köhler, Dr. Stephan Allgeier, Prof. Dr. Ralf Mikut
Karlsruhe Institute of Technology (KIT), Institute for Automation and Applied Informatics
Prof. Dr. Oliver Stachs
Rostock University Medical Center, Department of Ophthalmology
Key publications:
Allgeier S, Bartschat A, Bohn S, Guthoff RF, Hagenmeyer V, Kornelius L, Mikut R, Reichert KM, Sperlich K, Stache N, Stachs O, Köhler B. Real-time large-area imaging of the corneal subbasal nerve plexus. Sci Rep. 2022;12:2481. doi:10.1038/s41598-022-05983-5.
Sterenczak KA, Winter K, Sperlich K, Stahnke T, Linke S, Farrokhi S, Klemm M, Allgeier S, Köhler B, Reichert KM, Guthoff RF, Bohn S, Stachs O. Morphological characterization of the human corneal epithelium by in vivo confocal laser scanning microscopy. Quant Imaging Med Surg. 2021;11(5):1737-1750. doi:10.21037/qims-20-1052.
Bartschat A, Allgeier S, Scherr T, Stegmaier J, Bohn S, Reichert KM, Kuijper A, Reischl M, Stachs O, Köhler B, Mikut R. Fuzzy tissue detection for real-time focal control in corneal confocal microscopy. at-Autom. 2019;67(10): 879-888. doi:10.1515/auto-2019-0034.

